Do you have friends, relatives, or business partners worldwide and in different time zones? In this case, you probably need to know the daylight period worldwide. On World Route Planner’s daylight maps, you can determine if this is the right time to call them or if it is better not to.
Daylight Saving Time (or summer time as it is called in many countries) is a way of getting more light out of the day by advancing clocks by one hour during the summer. During Daylight Saving Time, the sun rises one hour later in the morning, when people are usually asleep anyway, and sets one hour later in the evening, stretching the day longer.
Daylight Saving Time Explained
Daylight saving is a summertime adjustment to the local time in a country or region, designed to cause a higher proportion of citizens’ waking hours to pass during daylight. To follow the system, timepieces are advanced by an hour on a pre-decided date in spring and reverted in the fall. About half of the world’s nations use daylight saving.
DST works because it saves energy due to less artificial light needed during the evening hours – clocks are set one hour ahead during the spring and one hour back to standard time in the autumn. Many countries observe DST, and many do not.
Note: Between March – April through September – November, it is summer in the northern hemisphere, where many countries may observe DST, while in the southern hemisphere, it is winter. The opposite is true during the rest of the year: winter in the northern hemisphere and summer in the southern.
Why We Have Daylight Saving Time
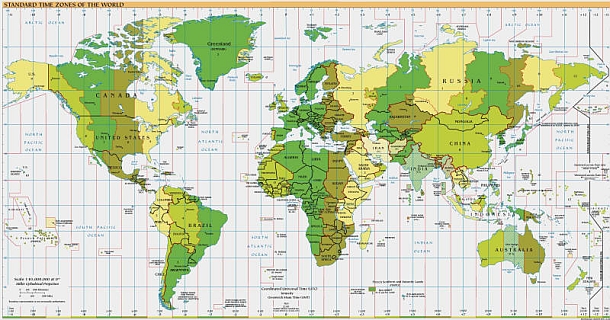
Click here to view World Time Zones.
Google’s interactive daylight map
The decrease in daylight during winter is due to the Earth’s axial tilt of 23 degrees. If the Earth were not tilted on its axis, the length of day and night would remain steady year long. During winter in the northern hemisphere, there are fewer daylight hours, and the southern hemisphere has longer daylight hours and experiences summer.
The Solar System
Nine significant planets, satellites, and countless minor planets (asteroids) orbit the Sun to form the Solar System. The Sun, our nearest star, creates energy from nuclear reactions deep within its interior, providing all the light and heat that make life on Earth possible. The Earth is unique in the Solar System in that it supports life: its size, gravitational pull, and distance from the Sun have all created the optimum conditions for the evolution of life.
The Sun
Diameter: 864,948 miles (1,392,000 km)
Mass: 1990 million million million million
The Sun was formed when a swirling cloud of dust and gas contracted, pulling matter into its center. When the temperature at the center rose to 1,000,000°C (1,800,000°F), nuclear fusion – fusing hydrogen into helium, creating energy – occurred, releasing a constant stream of heat and light.
The formation of the Solar System
The Sun threw clouds of dust and gas during its formation and cooled to form the solar system. The more minor planets nearest the Sun are formed of minerals and metals. The outer planets were formed at lower temperatures and consisted of swirling clouds of gases.
Solar eclipse
A solar eclipse occurs when the Moon passes between Earth and the Sun, casting its shadow on Earth’s surface. During a total eclipse, viewers along a strip of Earth’s surface called the area of totality see the Sun blotted out for a short time as the umbra (Moon’s full shadow) sweeps over them. Outside this area is a larger one, where the Sun appears only partly obscured as the penumbra (partial shadow) passes over.
The Incoming Energy From The Sun
The Earth receives energy from the Sun. This is termed “insolation”. Earth must return this energy to space if it is not to heat up. But the Earth is rotating; during the daytime, it receives energy, and it can radiate this energy back into space during the night. The Earth is also nearly spherical, so most of the energy received from the sun is around the equator, where the sun is overhead. In the polar regions, the sun is close to the horizon in summer and below the horizon in winter. The energy can be more effectively radiated back into space.
If the Earth were just a giant rock, the side facing the sun would become very hot during the day and very cold at night. Earth’s moon is just such an object. Its average distance from the sun is the same as the Earth’s. However, the day length of the moon is 29.5 Earth days (about 709 h). During the day, the average temperature of the moon’s surface is 107 °C (=225 °F), and where the sun is directly overhead, the temperature reaches 123 °C (=253 °F), well above the boiling point of water on Earth. During the lunar night, the average surface temperature drops to −153 °C (=−243 °F), with some areas falling to −233 °C (=−387 °F). These troubling facts escaped the producers of the 1984 film 2010—The Year We Make Contact, which shows the comfortable accommodations of our colony on the Moon. If the Earth were just a rock, it would also experience day-night temperature extremes, although they would be somewhat less because Earth’s day length is 24 h, so that during the day, there would be less time for the heat to build up and at night less time for the heat to dissipate. The greatest daily temperature ranges recorded at the Earth’s surface area in the Sahara (50 °C = 90 °F) and Siberia (67 °C = 120 °F). The night-day temperature range can be as small as 1 °C (=1.8 °F) in the tropics.
Fortunately, the Earth is not just a rock. Two fluids cover it: liquid water (the ocean, seas, and lakes) and a mixture of gases (the atmosphere). These fluids can circulate and redistribute the heat to reduce temperature contrasts on Earth’s surface. Some of the gases in the atmosphere absorb and reradiate energy. Transitions between the three phases of H2O, ice, water, and vapor, play a large role in redistributing energy on the Earth.
The incoming energy from the sun is called “short wave” radiation. Earth dissipates this back into space as “long wave” radiation, shown in Fig. 10.3. To determine how much and what wavelengths of radiation will be emitted by an object, physics uses Stefans’s Fourth Power Law and Kirchhoff’s “black body” concept. Remember that a black body is a hypothetical construct; it is an object that absorbs all the electromagnetic energy that falls onto it and transmits none of that energy. It is an ideal source of thermal radiation. There are no perfect “black body radiators” in the real world, but the sun and the tungsten filament in an electric light bulb come close. If the temperature of an object is below about 700 K (427 °C = 800 °F), the wavelengths emitted by it cannot be sensed by the human eye, and it appears black. However, if the object has a higher temperature, we see it as color. The sun’s surface temperature is about 5778 K (= 5,505 °C = 9,941 °F). Most of the energy received from the sun is in that part of the electromagnetic spectrum we call ‘light’, which ranges from shorter wavelengths we call violet or blue, to longer wavelengths we call red, as shown in Fig. 10.3. Some of the radiation is of even shorter wavelengths, the ultraviolet, and some are in longer wavelengths, the near-infrared. Human eyes have evolved to be sensitive to the most intense radiation from the sun. We cannot sense ultraviolet, probably because most of it does not reach the Earth’s surface. We can sense the infrared, which we feel as warmth or heat.
If you read the labels on light bulbs or do much color photography, you are already familiar with this temperature-radiation effect. When you replace your incandescent bulbs with fluorescent or LED bulbs that use much less energy, you will find the color of the light emitted by the bulb described as some temperature Kelvin. For a warm candlelight feel, you want about 4,000 K; for a daylight effect, you want 5,700 K, of course; and for garnish blue-white light, you want 7,000 K. some excellent sites on the web will show you what the color of the light emitted from the bulb will be. In fluorescent bulbs, it is not a heated filament that emits the light; the fluorescent bulb is no ‘black body’ but uses a wholly different principle to emit light—that’s why it uses so much less energy and doesn’t get hot. You can even buy fluorescent bulbs that will work with a dimmer. Their color temperature stays almost the same as dimmed; they emit less light. If you dim an incandescent light bulb, it goes from white to orange to red.
Solar Variability
The total energy received each year by Earth will change if the luminosity of the Sun changes. If the Sun emits more energy, the Earth must warm. Unfortunately, we have only a brief, direct history of changes in solar radiance. The question sometimes arises – could our Sun be a variable star with significant changes in its energy output over time? The “variable stars” astronomers speak of changes in brightness from hours to days to a few years. We do not have a long enough astronomical record of how they might vary over centennial or longer timescales.
On the other hand, we know about variations in our Sun’s radiance on decadal and centennial time scales from direct evidence. There are also some indications that it may vary on thousand-year time scales. Finally, there are climatic variations on millennial time scales attributed to the Sun without any real evidence for a link.
Our Sun is a variable star, with sunspots appearing and disappearing on a roughly 11-year cycle. The Sun radiates more energy when sunspots are present than when they are not. This is because “faculae”, bright spots among the dark sunspots, more than makeup for the lowered radiation from the spots. The change in insolation between sunspot maxima and minima is about 0.5 W/m2, a difference of less than 0.1 %.
There have been periods when there were no sunspots for several decades. The most recent was the Maunder Minimum, which lasted from 1645 to 1710. The Maunder Minimum coincided with an especially cold phase of the “Little Ice Age,” It has been suggested that diminished insolation may have been the cause. However, other possibilities have been proposed recently. Other inconclusive attempts to relate sunspot activity to global climate, wine vintages, and economics.
Satellite observations since 1978 have documented solar variability with a precision previously unknown. They have shown that while the variations of the visible spectrum with the sunspot cycle are very small, they may be as much as 50 % in the ultraviolet.
Solar storms are events on the Sun that produce flares and eject matter from its corona. The solar storm of 1859, the Carrington Flare, disrupted the then-new telegraph lines and other electrical devices.
Cosmic rays are highly energetic particles, about 90 % protons, and the rest, mostly helium nuclei, travel at very high speeds. They come from outside our solar system. They can be deflected by particles that form the solar wind from the Sun’s outer atmosphere, the corona. The solar wind’s strength varies with the sunspot cycle and induces an 11-year cycle in cosmic ray intensity. As they approach the Earth, its magnetic field can deflect cosmic ray particles.
In the 1930s, it was discovered that cosmic rays produced showers of subatomic particles into the lower part of the atmosphere. These air showers result from the impact of highly energetic incoming particles with molecules in the upper atmosphere. Billions of exotic, short-lived particles are produced in these collisions and rain down into the lower levels of the atmosphere. After WWII, the investigation of cosmic rays and the advent of high-energy particle accelerators led to the discovery of new kinds of subatomic particles: more kinds of muons, pions, kaons, and neutrinos. There are more than 25 kinds of subatomic objects in the ‘particle zoo.’
Two of the byproducts of cosmic ray interactions are carbon-14 and beryllium-10. Carbon-14 is formed from nitrogen-14 as one of the protons in its nucleus is replaced by a neutron. It has a half-life of 5,730 years and has been widely used for dating materials back to 20,000 years; more recently, with the improved technology of accelerator mass spectrometry, back to 60,000 years. Carbon-14 dating is complicated because the rate of its production in the upper atmosphere varies with time and because of contamination of the atmospheric reservoir by ‘dead’ carbon introduced by burning fossil fuels. These problems have been overcome for the younger part of the record by comparing carbon-14 dates with tree ring ages.
Beryllium-10 is a spallation product produced by cosmic ray particles splitting atoms in the molecules of the air or materials in the ground. It has a half-life of about 1.5 million years and has been used to determine the changes in the rate of carbon-14 production in the upper atmosphere.
There has been speculation that the ionization of air molecules by cosmic rays may create nucleation sites for water droplets and influence cloud formation, thus impacting Earth’s climate. This speculation was prevalent briefly during the late 1990s and early 2000s. The evidence for correlations has not stood up to careful examination.
The energy from the interior of the Earth is only about one-fourth of a percent of the insolation, much too small to have any effect on climate.
Click here to view World Time Zones.